38 fda approved health claims on food labels
Understanding Health Claims on Food Labels - Food Smart Colorado Health Claim Definitions In addition to the nutrition facts and ingredient information found on packaged foods, some foods may also be labeled with health-related claims. There are three main categories of claims defined by and regulated by the Food and Drug Administration (FDA): Nutrient Content Claim vs Health Claim - LabelCalc Nutrient content claims, which are commonly used on food labels, either refer to the amount of a nutrient in a product or compare the levels of a nutrient in that food to a similar reference food. ... According to the FDA, health claims refer to the relationship between a specific food product or ingredient and a reduced risk of disease or a ...
Health Claims on Food Labels - LabelCalc Health claims, according to the FDA, are statements about the relationship between a food product or ingredient and a reduced risk of disease or a health condition. Basically, the FDA distinguishes two kinds of health claims: "authorized" and "qualified.". Authorized Health Claims: Claims that have significant scientific agreement (SSA).

Fda approved health claims on food labels
Label Claims for Conventional Foods and Dietary Supplements | FDA there are three ways in which fda exercises its oversight in determining which health claims may be used on a label or in labeling for a conventional food or dietary supplement: 1) the 1990... Structure/Function Claims | FDA If a dietary supplement label includes such a claim, it must state in a "disclaimer" that FDA has not evaluated the claim. The disclaimer must also state that the dietary supplement product is not... Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims,...
Fda approved health claims on food labels. Code of Federal Regulations Title 21 - Food and Drug Administration For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 101.14 Health claims: general requirements. (a) Definitions. For purposes of this section, the following definitions apply: (1) Health claim means any claim made on the label or in labeling of a food, including a dietary supplement ... The Basics of Food Product Health Claims - LabelCalc For additional information from the FDA on Health Claims, Download this PDF and scroll to Appendix C. Claim #3: Qualified Health Claims. Qualified food product health claims refer to a claim made on a food product label in reference to a disease that does not have to meet the rigorous standards of an Authorized Health Claim. Qualified Health Claims | FDA Food manufacturers can petition the agency to consider exercising enforcement discretion for the use of a qualified health claim. The FDA does not "approve" qualified health claim petitions. Nutrient Content Claims | FDA Nutrient Content Claims. See Claims That Can Be Made for Conventional Foods and Dietary Supplements for definitions of claims. Final Rule: Food Labeling: Nutrient Content Claims; Alpha-Linolenic ...
Food labeling: health claims; plant sterol/stanol esters and coronary ... The Food and Drug Administration (FDA) is authorizing the use, on food labels and in food labeling, of health claims on the association between plant sterol/stanol esters and reduced risk of coronary heart disease (CHD). FDA is taking this action in response to a petition filed by Lipton (plant ster … FDA Permits Qualified Health Claims on Nut Labels For the first time, the U.S. Food and Drug Administration has recognized a qualified health claim made by a conventional food. In a surprise move in July the FDA granted permission for peanuts, almonds, hazelnuts, pecans, pistachios and walnuts to carry a label touting their heart-healthy effects. FDA Food Labeling Enforcement - LabelCalc FDA food labeling enforcement. Online nutrition analysis software makes it easy to create FDA-compliant nutrition labels. ... You can then choose from a variety of FDA-approved label formats and sizes to find one that suits your package, ... health claims, or to simply highlight certain nutrients that your product is rich in. Of course, if you ... A Guide to FDA Regulation of Food Labeling Claims Among the FDA-regulated claims commonly declared on food labels are nutrient-content claims, health claims, qualified health claims and structure/function claims. Additionally, FDA has authority over claims related to gluten content, genetically modified organisms (GMOs) and "natural."
Enforcement Policy Statement on Food Advertising In addition to requiring nutrition information on virtually all food products, the NLEA directed FDA to standardize and limit the terms permitted on labels, and allows only FDA-approved nutrient content claims and health claims to appear on food labels.7 While the NLEA is designed in part to prevent deceptive and misleading claims on labels ... What are some examples of an FDA health claim on a food label? Likewise, people ask, what health claims are allowed on food labels? Approved Health Claims Calcium, Vitamin D, and Osteoporosis. Dietary Lipids (Fat) and Cancer. Dietary Saturated Fat and Cholesterol and Risk of Coronary Heart Disease. Dietary Non-cariogenic Carbohydrate Sweeteners and Dental Caries. Authorized Health Claims That Meet the Significant Scientific Agreement ... Authorized Health Claims That Meet the Significant Scientific Agreement (SSA) Standard Authorized health claims in food labeling are claims that have been reviewed by FDA and are allowed on food... Questions and Answers on Health Claims in Food Labeling | FDA All health claims, whether authorized or qualified, require pre-market review by the FDA. Under federal law, the FDA approves by regulation authorized health claims for use in food labeling only if...
Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims,...
Structure/Function Claims | FDA If a dietary supplement label includes such a claim, it must state in a "disclaimer" that FDA has not evaluated the claim. The disclaimer must also state that the dietary supplement product is not...
Label Claims for Conventional Foods and Dietary Supplements | FDA there are three ways in which fda exercises its oversight in determining which health claims may be used on a label or in labeling for a conventional food or dietary supplement: 1) the 1990...









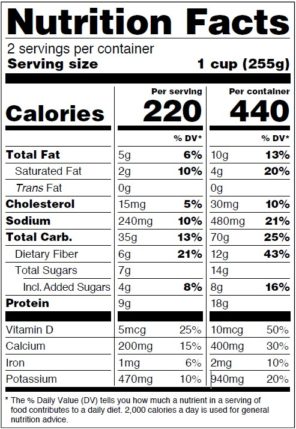
Post a Comment for "38 fda approved health claims on food labels"